Measuring Oxidation in Extra Virgin Olive Oil
admin | January 21, 2016This is the 3rd of a short series aimed at explaining the whys, the how and the what (and the what-nots) of analyses that are commonly applied to extra virgin olive oil. This time, I present what I think is a reliable indicator of olive oil oxidation – K232.
FACT FILE
Name: K232 (short for absorbance at 232 nanometers).
What does it measure?: Level of oxidation.
How does it work? All olive oils contain some polyunsaturated fatty acids. These have a basic chemical structure that has two chemical double bonds amongst a long line of single chemical bonds. In an unoxidised state, the pattern of bonding is (Double-Single-Single-Double). But during oxidation of the fatty acid, the conga line of bonds changes to (Double-Single-Double Single).
Whilst this change in order might seem inconsequential, the oxidized rearrangement (D-S-D-S known as a ‘conjugated diene’) has a particular property in that (unlike its unoxidised D-S-S-D cousin), it strongly absorbs ultraviolet light at a specific wavelength of 232 nanometers.
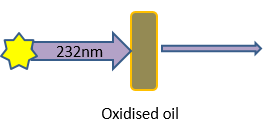
So shining a beam of UV light at 232nm at an oil and measuring how much of it is absorbed can be used as a measure of the oils oxidation status. This is done using a peice of scientific equipment called a spectrophotometer. A schematic as to how the analysis works is shown in Figure 1.
Figure 1: A schematic showing the basic principle behind the K232 analysis for oxidation.
Unit of Measurement: absorbance units(au).
Does it affect extra virgin olive oil status?: Yes. To be extra virgin, the oil must absorb at less than 2.55 absorbance units at 232nm. In my opinion 2.55 is (like most of the IOC limits), way too lax. Most 2 year old oils can make the grade pretty comfortably. But if that is what they want……
Is it a measure of quality?: Yes. K232 has been shown to be a good predictors of the freshness of extra virgin olive oil in Australian competitions (Australia is the only country that routinely measures the chemistry of competition entries).
Official method degree of difficulty: Easy, but for accuracy, the oil sample needs to be accurately diluted by a technician with a relatively expensive solvent so the cost is moderate. It can be measured using cheaper NIR (Near Infrared Spectroscopy) method. However, like all NIR analysis, the validity of the accuracy depends squarely on the quality of the calibration available to the laboratory.
Typical effects of high readings of UV 232
– The oil displays tiredness and in some cases rancidity.
– Shorter shelf life.
K232 level in extra virgin olive oil is increased by:
– Delays between harvest and processing, incidence of fungal disease, olive fly attack and frost damage increase K232.
– Age.
– Oxidative bottling practices.
– Poor storage conditions.