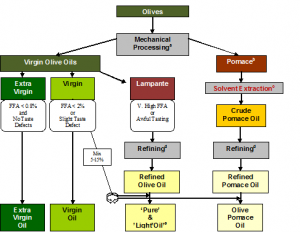
Challenging the Unchallengable
admin | January 26, 2014Each year I attend the Australian National Extra Virgin Olive Oil Conference hosted by the Australian Olive Association, where Tim Smith, Marketing Manager for Australia’s largest extra virgin olive oil producer, Boundary Bend Limited (known to most by their brand, Cobram Estate), usually presents an update of the trends in the Australian olive oil market.
His informative presentations draw on a significant amount of data that either isn’t either publicly available (i.e supermarket sales data from private market research firms), or data that takes quite a bit of time compile such as imports and exports. I always look forward to hearing about how Australian producers are faring in their quest to gain greater consumer acceptance, and in wrestling a greater share of the domestic olive oil market from their powerful EU competitors.
So during his presentation given in November 2013 in Hobart, Tasmania, I scribbled down as many of the interesting factoids that I could with the view to sharing what I found particularly interesting.
The message? It IS possible for New World producers to wrest market share from the powerful EU packagers, if they efficiently produce consistently high quality extra virgin olive oil at a competitive price.
Having said that, the business of selling olive oil is a game of thrust, parry and riposte. Traditionally market share has been wrested back by undercutting the competition, which is much easier to do when your commercial operations are propped up by Governments (aka taxpayers) via payment of production and storage subsidies, and import tariffs are levied on the competition. Australia has no import duties/tarriffs on olive oil entering from the EU (or anywhere else), and, unlike the EU, Australian producers do not receive production or storage subsidies from taxpayers. These free trade policies (which I support by the way) unfortunately leave the progress made by local producers vulnerable to being wound back by those not subject to the same level playing field.
I guess time will tell, but I’m confident that quality at a competitive price will continue to put good old fashioned competitive pressure on those who thought they would never be challenged.
You can view the presentation in full screen mode at here (use the arrow keys at the bottom to navigate).